Is Caco3 Soluble In Water
Structure & Reactivity in Chemistry
Structure-Property Relationships
SP10. Solubility and Ions
In order for two substances to exist miscible, or in order for one substance to be soluble in another, the two kinds of molecules must interact with each other. Not only that, but at that place must exist strong enough attraction of one type of molecule for the other then that they are willing to move away from their identical siblings. If the attractive forces between the different molecules are strong plenty to overcome those forces between both sets of like molecules, dissolution results.
Table salt dissolves in water because the very polar water molecules attract both the positively charged sodium ions and the negatively charged chloride ions. This interaction is chosen an ion / dipole interaction.
In club to distribute the sodium cations and chloride ions throughout the solution, some of the h2o molecules may need to back away from each other to brand room for the ions. Some of the strong hydrogen bonds may be lost, but the energy needed to overcome those interactions is compensated by the attraction between the dipole of the water and the sodium or chloride ions.
Other salts deliquesce in water, too, but some of them deliquesce more hands than others. You lot could easily dissolve well-nigh 360 thousand of table table salt in a liter of water, but the solubility of calcium carbonate is only about 0.01 grams per liter. That's partly due to the fact that the ions in sodium chloride, Na+ and Cl-, take lower charges than the ions in calcium carbonate, Ca2+ and COiii 2-. The higher the charges on the ions, the stronger their electrostatic allure for each other, and the harder it is for the water to pull them apart. It's a skilful thing, besides; if the calcium carbonate in marble were to deliquesce too easily, retrieve of how many buildings and statues would deliquesce in the rain.
Problem SP10.one.
Rank the following salts from near hands to least easily dissolved in h2o: MgSOiv, LiCl, AlPO3
The factors that command the solubility of ions can be circuitous, though. For instance, the charges on the ions too affect the strength of the interaction with the water molecules. Highly charged ions interact more than strongly with each other, only they also interact more strongly with h2o molecules.
In that location are other solvents that tin can dissolve salts via ion / dipole interactions. Typically they would have strong dipoles. However, not many solvents take dipoles as strong as that of water.
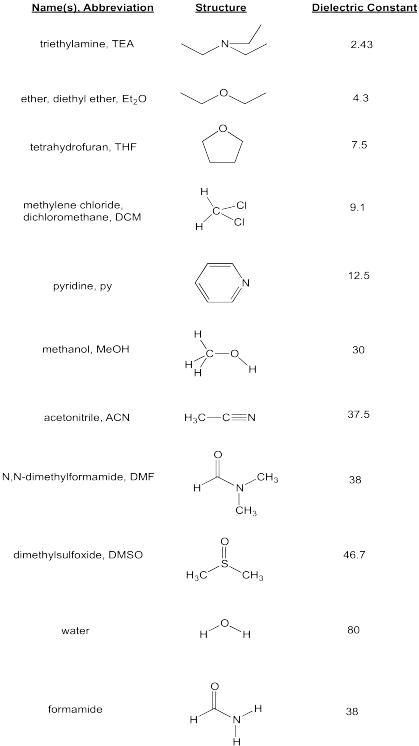
Problem SP10.ii.
Which of the post-obit solvents would be most capable of dissolving some LiCl? Rank from greatest solubility to least solubility.
triethylamine, dimethylsulfoxide, dichloromethane, acetonitrile, pyridine.
Of course, it isn't just salts that tin dissolve in solvents. Other solids tin can, too, if they can interact with solvent molecules strongly enough. Sugar also dissolves pretty well in water. Why exercise you think that is so?
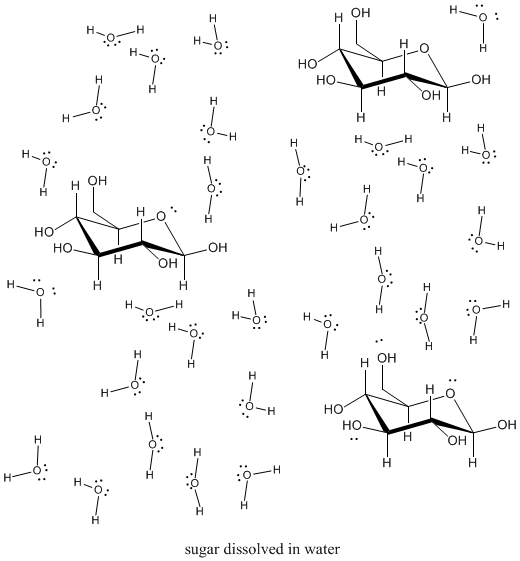
Sugars, of course, tin can hydrogen bond hands with h2o, so they are usually pretty soluble.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John'due south University (with contributions from other authors as noted). It is freely available for educational use.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Eatables Attribution-NonCommercial three.0 Unported License.
Ship corrections to cschaller@csbsju.edu
Dorsum to Structure & Properties: Intermolecular Attractions
Back to Web Materials on Structure & Reactivity in Chemical science
Is Caco3 Soluble In Water,
Source: https://employees.csbsju.edu/cschaller/Principles%20Chem/imf/SPsolubility.htm
Posted by: nicholstheacce.blogspot.com


0 Response to "Is Caco3 Soluble In Water"
Post a Comment